The Phosphorus Cycle
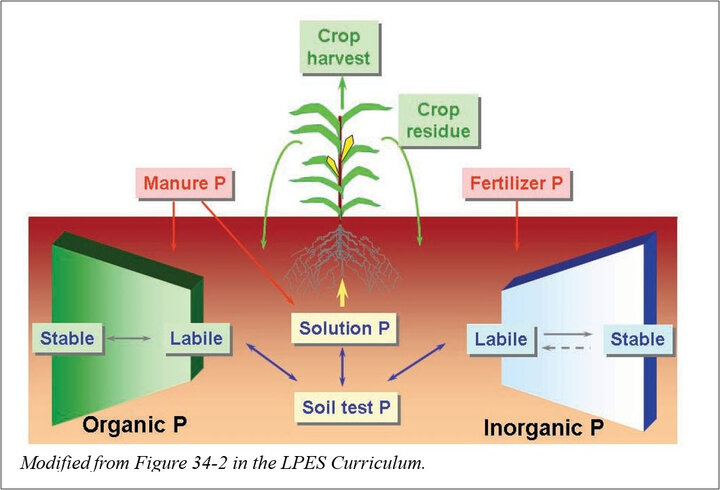
Animal manures contain both organic and inorganic forms of phosphorus. When manure mineralizes, organic phosphorus becomes inorganic phosphorus in solution and is available to plants. Some organic phosphorus is transformed to inorganic form shortly after application but other phosphorus will remain in organic form. Soil organic phosphorus consists of labile and stable fractions. The labile fractions will be mineralized after a short time while the stable fractions may remain in organic form for years.
Solution phosphorus is in the form of anions that react with cations such as iron, aluminum, and calcium to become attached phosphorus and unavailable to plants. Attached phosphorus may be loosely bonded (labile phosphorus), or tightly bonded in the soil (non-labile or stable phosphorus). The rate of reaction of dissolved phosphorus anions and compounds formed depends on soil pH. A greater proportion of the total soil phosphorus is likely to be bioavailable, or labile phosphorus, between pH 5.5 and 7.0 than at higher or lower pH.
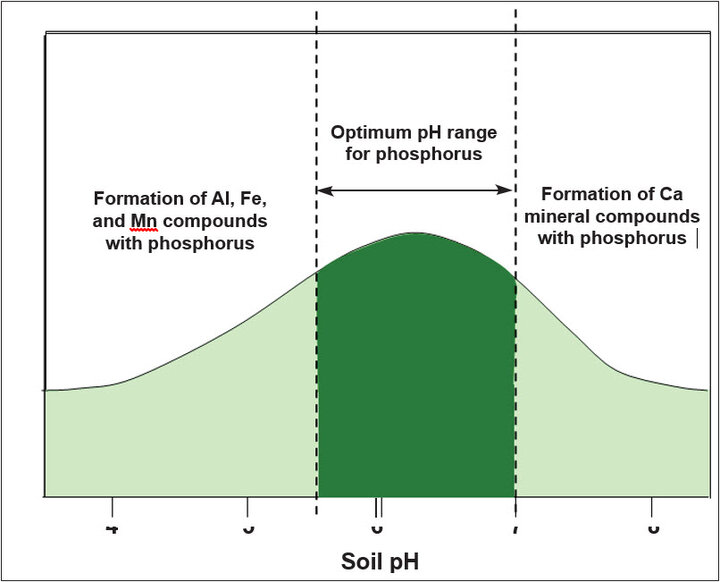
At low soil pH, dissolved phosphorus quickly reacts with soluble iron, aluminum, and manganese to form precipitates, or it is adsorped by hydroxides of iron and aluminum and by other clay minerals. Under moderately acid conditions, chemical precipitation of phosphorus is unlikely, but much dissolved phosphorus becomes adsorped. At high pH, dissolved phosphorus reacts with calcium to precipitate as calcium phosphate. The potential for phosphorus adsorption also increases as the clay content of soil increases.
Phosphorus or Phosphate?
Phosphorus content in soil, plants and animal rations is expressed as phosphorus (P) content, but phosphorus in fertilizers and manure intended for land application is expressed as phosphate (P2O5).
- To convert P to P2O5 concentration, multiply by 2.29
- To convert P2O5 to P, multipy by 0.44
With repeated application of manure, available soil phosphorus can become excessive with high potential for runoff. Soil phosphorus leaches very slowly, but when the water table is high and the soil is sandy, leaching of phosphorus to groundwater may be of concern.
Soil phosphorus build-up
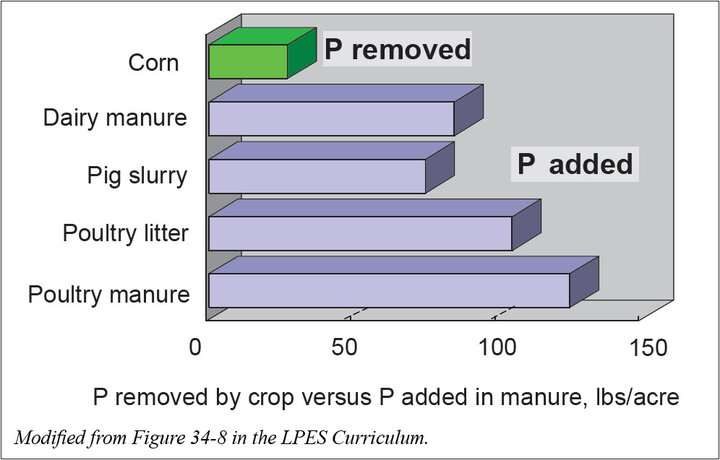
Crops typically remove two to five pounds of nitrogen for every pound of P2O5 in the harvested product. In contrast, slurry and solid manures typically supply 0.9 to 1.5 pounds of nitrogen for every one pound of P2O5 ; the ratio contained in liquid manures is similar to that of crops. Applying manure to meet the nitrogen requirement of plants often results in excessive application of phosphorus. Application of manure to supply 200 pounds/acre of nitrogen may supply about 130 pounds/acre P2O5 in beef manure or about 200 pounds/acre P2O5 in swine or poultry manure.
Soil phosphorus losses to water bodies
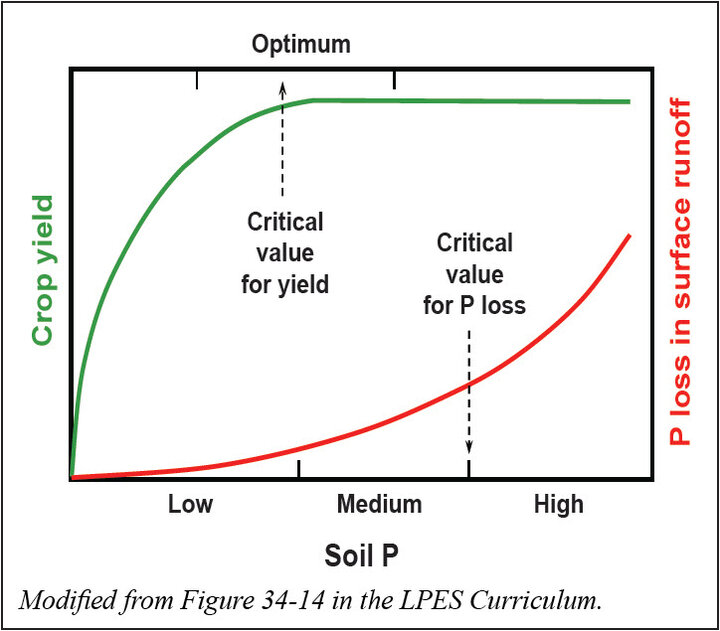
Agricultural byproducts are the greatest contributor of phosphorus to surface waters in much of Nebraska. In order to minimize the transport costs for manure disposal, manure is often applied to land at excessive rates near the animal feeding operation. Repeated applications of manure to meet nitrogen needs can result in an increase in soil phosphorus levels that may pose significant risk of surface water contamination. Phosphorus levels in the upper surface soil are likely to be much higher following manure application than the average for soil sampled to an 8-inch depth. This is especially true if manure is not well incorporated into the soil. As available soil phosphorus levels increase beyond the critical value for crop yield, the potential for phosphorus loss increases (see graphic to right). Several factors contribute to the potential for phosphorus loss from land to water bodies.
Part I of Phosphorus Dynamics | Part II - Phosphorus Loss | Complete the Quiz